- Product Details
Keywords
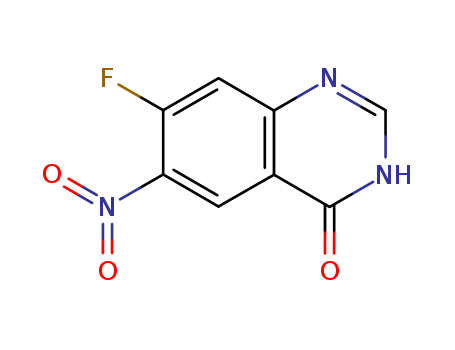
- 7-FLUORO-6-NITRO-4-HYDROXY-QUINAZOLINE
- 162012-69-3
- Pharmaceutical Intermediate
Quick Details
- ProName: 7-FLUORO-6-NITRO-4-HYDROXY-QUINAZOLINE
- CasNo: 162012-69-3
- Appearance: White or almost white crystalline powd...
- Application: Pharmaceutical Intermediate
- DeliveryTime: Stock or 1-2 weeks
- PackAge: Based on customer needs
- Port: Any port in china. Based on customer ...
- Purity: 99.0%, High purity.
- Storage: Store in a dry and cool place
- Transportation: By Sea/Air. Based on customer needs
- LimitNum: 0
- Moisture Content: Execute according to product standards...
- Impurity: Execute according to product standards...
Superiority
High quality. Factory supply. In stock. Best price.
1.Quick response within 24 hours;
2.Best quality in your requirement;?
3.We pay more attention on delivery time, and usually ship on time;
4.Under the premise of safety and effectiveness, we can produce a large number of products quickly;
5.Free samples will be provided, Ensure specifications and quality are right for customer;
6.Customers will receive the most professional technical support and efficient service;
Please send the quantity or weight to us, we will arrange our sales to provide the one-for-one service for you.
For more information about us.
Our company is located in Anqing High-tech Industrial Development Zone (a national chemical park) in Anhui province, covering an area of 20,000㎡, with two three-storey Class A plant buildings including 6 workshops: 2 of them are used for the manufacturing of High-end pharmaceutical intermediates, and the rest 4 workshops are GMP workshop, which are utilized to provide MAH services.
We also set an R&D center in JSLS at No. 9 Weidi Road in Nanjing city, with a total land of 1,800㎡. We have an experienced and energetic R&D team equipped with advanced organic synthesis and analytical testing apparatus. Also we areequipped with the data audit trail system (online version) and ISO9001 quality certificated management system which can help us meet the regulatory requirements for API registration and declaration.
ANQING CHICO PHARMACEUTICAL CO.,LTD. exported this product to many countries and regions at best price. If you are looking for the material's manufacturer or supplier in china, CHICO is your best choice.
Details
Through the successful development of tinib, liptin, liflozin and xaban products, our company has established closely cooperative relations with more than 30 of the top-100 pharmaceutical companies in China to help Chinese generic drugs research, development and marketing. In addition, we also vigorously expand the overseas market, with Asia, North America, South America, Europe and other countries to establish cooperative relations, and passed the relevant supplier qualification audit.
As patents expire and generic drugs come on the market, some projects have successfully achieved commercial mass production, and new projects will be approved for marketing every 1-2 years which will give power to the sustainable development of our company.
Regarding quality as number one priority, we have established a strict quality assurance system reference to GMP requirements. In accordance with the requirements of relevant EHS laws and regulations of China, we also have established a sound EHS management system and strive to create a safe, healthy and clean working environment for our employees to achieve sustainable development.
The listed products which involve patents, are only for research and development ,and not used for sales.Regulated products will be sold in strict accordance with the laws of China and the laws of the buyer's country.All products are not for human use ,and all risks associated with the sale to the country that constitutes a patent infringement shall be borne by the buyer.?
Disclaimer:Products are only available to countries where there is no valid patent protection. Products still covered by patents rights are available exclusively for experimental or registration purpose pursuant to national applicable law, and shall be sold in strict accordance with the laws of the People's Republic of China and the laws of the Buyer's country. All products are not for human use. Buyer is obligated for evaluation of the patent situation in its domestic market and shall be held liable for uses which do not fall within the scope of the experimental or registration use exception and are not permitted by national applicable law.


 Premiumsupplier
Premiumsupplier